Difference between Electrons and Protons
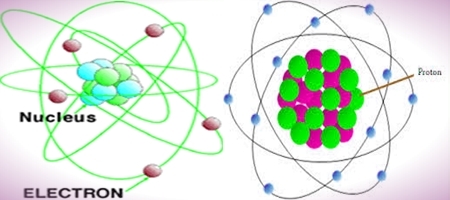
Difference between Electrons and Protons
Atom is a Greek word which means “Undividable”. And it was invented John Dalton in 1803. Atom is composed of Electrons, Protons and Neutrons. Neutrons and Protons are present inside the nucleus of atom. And the Electrons are present outside the nucleus and revolve around the nucleus in orbits which are also known as shells.
Electrons
Electrons were invented by Joseph John Thomson in 1897. He was awarded with Nobel Prize on his work on Electrons. In 1897 he performed many experiments which show the presence of negatively charged particles outside the nucleus which were named electrons. Electrons are Greek word which means yellowish-brown.
Protons
Protons were discovered by Ernest Rutherford in 1920 on experimental basis. Ernest Rutherford was specialist in field of Physics and Chemistry. It is present inside the nucleus of Atom and is positively charged. Protons are named after a Greek word which means “Main”.
Electrons VS Protons
Charge:
Negative charge is present on Electrons.
Positive charge is present on Protons.
Presence:
Electrons revolve around the nucleus which means they are circling in orbits which are also called as shells. The number of electrons is known as Atomic Number.
Protons are existent inside the nucleus. And along with Neutron they form Atomic Mass.
Mass:
The Mass of Electron in grams is 9.110 x 10-28 g, the mass of Electron in electron volt unit is
0.51099906 MeV and in atomic mass unit the mass is 0.000549amu.
The Mass of Electrons in grams is 1.6727 x 10-24g, the mass of Proton in electron volt unit is 938.27231 MeV and in atomic mass unit the mass is 1.007316amu.
Symbol:
Electrons are represented by e- or β−.
Protons are represented p, p+ and N+.
Usage:
From the number of electrons the chemical properties such as boiling point, stability and conductivity can be predicted.
By using the number of protons the chemical properties of atom can be determined and atoms are arranged according to the growing atomic number.
Size:
The Electron is less gigantic than protons.
The Proton is 1835 times more massive than electron.
Example:
The number of Electrons of sodium Na is 11.
The number of Protons of sodium Na is also 11.
Conclusion
Electron and proton have opposite charge and they attract each other. The number of electrons and protons are equal. The number of electron along with neutron forms Atomic Mass. And two protons and electrons deter each other. On the basis of number of protons and electrons the atoms are arranged in the periodic table.


Leave a Reply